Here are two powerful tools that can make interactive communications with FDA more efficient if your device is eligible.
Breakthrough Devices Program
The Breakthrough Devices Program is a voluntary program applicable to medical devices and device-led combination products that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. It is available for devices and device-led combination products which are subject to review under a premarket approval application (PMA), premarket notification (510(k)), or De Novo classification request (“De Novo request”).
The goal of the Breakthrough Devices Program is to help patients have faster access to medical devices by making the regulatory process faster without altering the legal and ethical requirements that govern FDA’s mission to protect and promote public health.®
The Breakthrough Devices Program is comprised of two phases.
Phase 1: The first is the Designation Request phase, in which an interested sponsor of a device requests that the FDA grant that device Breakthrough Device designation.
Phase 2: After being admitted to the Breakthrough Device Program, FDA works with the sponsor to expedite the development of the device by supporting prioritized reviews and interactive feedback.
Eligibility:
Breakthrough Device Program criteria are as follows:
- Mandatory: Device provides more effective treatment or diagnosis of life-threatening or irreversibly debilitating human disease or conditions
- Also, your device must meet at least one of the following:
- The device represents breakthrough technology (either novel technology or a novel application of current technology)
- No approved or cleared alternatives exist
- The device offers significant advantages over existing alternatives
- Device availability is in the best interest of the patient
Advantages of Breakthrough Designation:
If your device obtains a Breakthrough Designation, you have many advantages in working with FDA including:
- Clinical study design support
- FDA Review Team Manager: An FDA manager in that unit will assess the submission and assign the most appropriate individual to lead the review team based on training, expertise, experience, and the ability to quickly and interactively resolve complex issues
- Senior FDA management will be involved in the review process
- Priority review: All submissions for devices designated as Breakthrough Devices will receive priority review, meaning that the review of the submission is placed at the top of the appropriate review queue and receives additional review resources, as needed.
How to request admission to the Breakthrough Device Program:
To see if your device is eligible for the Breakthrough Device Program, you need to create a briefing document which is submitted via the Q-Submission process. We typically create a document with the following sections minimally:
- Device description
- Indications for use
- Regulatory history
- How device meet Breakthrough Device criteria
- Type of marketing submission (510(k), De Novo, PMA)
When to submit:
The whole point of this program is to be able to interact with FDA more quickly and more often, so the designation request should be prior to critical regulatory submissions.
Safer Technologies Program (STeP)
STeP is essentially a sister-program to the Breakthrough Device Program where the device is reasonably expected to significantly improve the safety of currently available treatments or diagnostics that target an underlying disease or condition that are less serious than those eligible for the Breakthrough Devices Program. Unlike the BDP, devices eligible for STeP may include devices that are intended to treat or diagnose diseases or conditions that are non-life-threatening or reasonably reversible. STeP is modeled after the Breakthrough Devices Program and can have similar benefits. However, Breakthrough is considered more critical than STeP meaning STeP designated devices receive extra support from FDA as resources allow.
Eligibility:
Eligibility Requirements for the STeP program are as follows:
- Not eligible for Breakthrough designation
- Device is expected to improve the benefit-risk profile of a treatment or diagnostic through substantial safety innovations that provide for at least oneof the following
- a reduction in the occurrence of a known serious adverse event
- a reduction in the occurrence of a known device failure mode
- a reduction in the occurrence of a known use-related hazard or use error
- an improvement in the safety of another device or intervention
Advantages of Safer Technology Program:
The advantages of the STeP program are similar to the Breakthrough Designation Program however STeP devices are lower on FDA’s priority list and are more subject to resource constraints.
How to request admission to the Safer Technology Program:
The process is as simple as submitting a Q-Submission requesting inclusion in STeP with this request highlighted in the cover letter. The following are typical sections of a submission:
- Device Description
- Proposed indications for use
- Expected safety improvement
- Regulatory history
- How device meets the STeP criteria
- Type of marketing submission (510(k), De Novo, PMA)
When to submit:
As with the Breakthrough Designation Program, a sponsor should apply prior to upcoming FDA submissions in order to take advantages of the program if accepted.
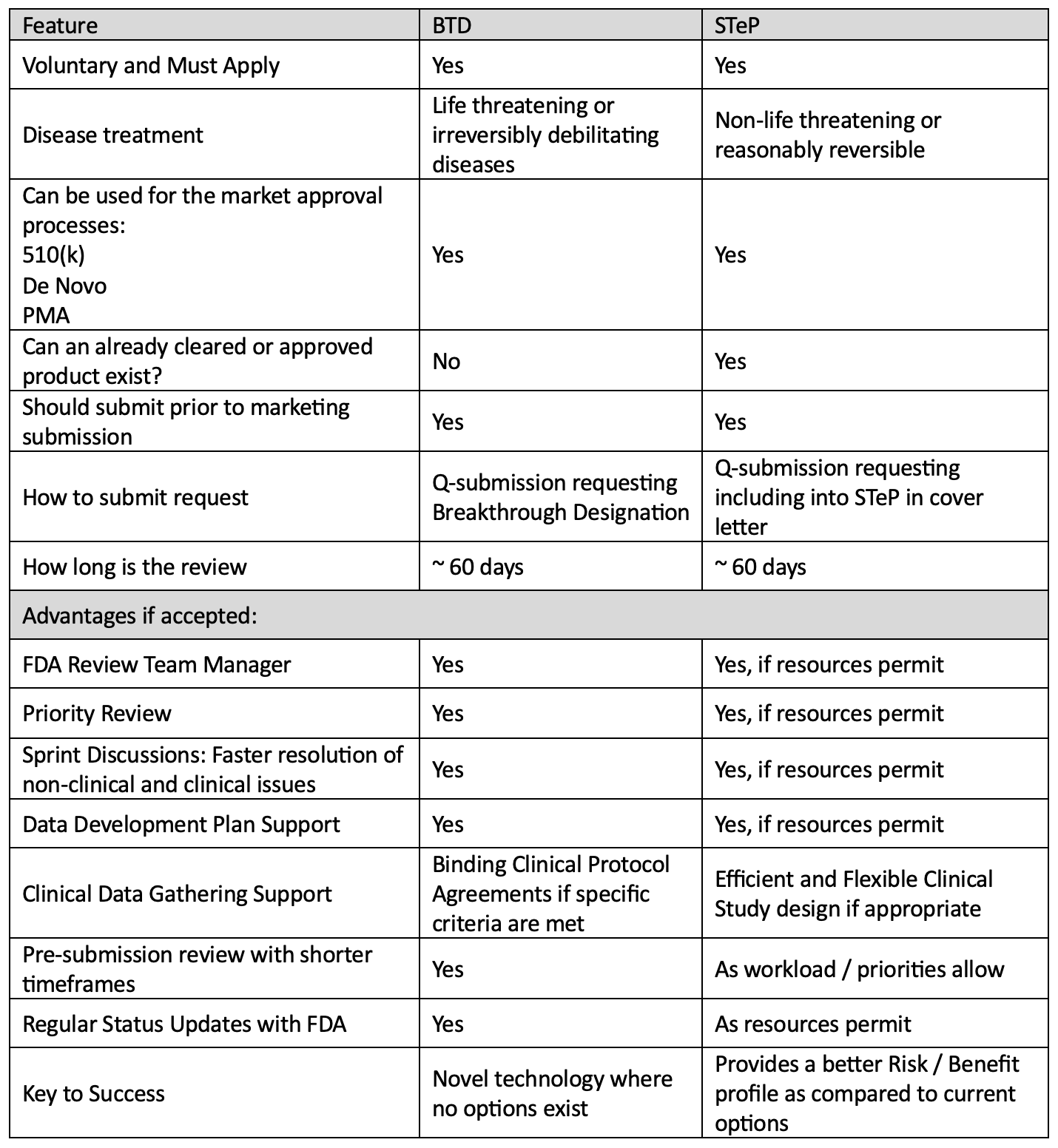
Comparison: Which Pathway is Best for You?
Both the Breakthrough or Safer Technology designation can give a medical device company a competitive advantage by allowing more streamlined interactions with FDA. The following table provides general guidelines which can help you select the right program for your device.
References
FDA Guidance: Safer Technologies Program for Medical Devices January 6, 2021 https://www.fda.gov/media/130815/download
FDA Guidance: Breakthrough Devices Program September 15, 2023
Bring Your Medical Device to Market Faster



